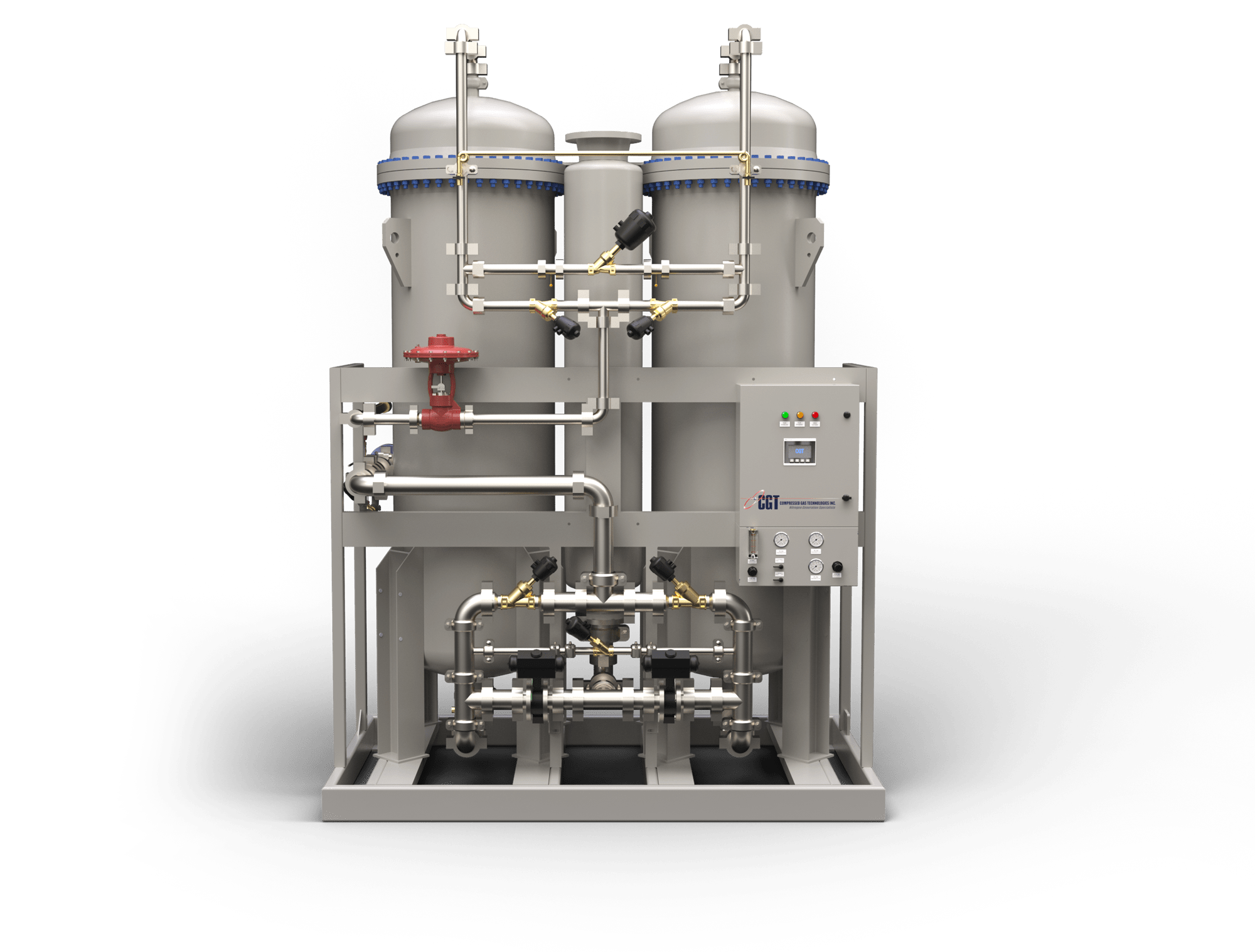
Pharmaform invests in custom built PMNG membrane nitrogen generators from CGT

Pharmaform has ordered two custom built PMNG membrane nitrogen generators from Compressed Gas Technologies. The nitrogen generators will be used in Pharmaform’s new aqueous and solvent spray drying system. Pharmaform chose CGT because of its capabilities to custom build equipment to their specifications as well as the short lead time.
Austin, TX, November 4, 2009 – PharmaForm, a contract pharmaceutical development and manufacturing organization and a wholly owned subsidiary of Akela Pharma Inc. (TSX: AKL), today announced that it has added aqueous and solvent spray drying to its portfolio of bioavailability enhancement solutions. A widely used processing technology, spray drying offers a number of benefits to optimize drug formulation, including enhanced bioavailability of poorly soluble compounds, higher drug loading and predictable scale-up.
“PharmaForm’s investment in the Anhydro MicraSpray 35® is part of the company’s strategic focus and commitment to provide comprehensive solutions for poorly soluble compounds,” said Gregory M. McKee, president and chief executive officer of Akela Pharma. “Spray drying further enables us to better serve our clients by offering another proven, commercializable technology to enhance bioavailability and accelerate their drug development programs.”
In addition to the new spray drying capability, PharmaForm’s comprehensive bioavailability enhancement technologies include hot-melt extrusion, solvent-based fluid bed processing and liquid filled hard capsules.